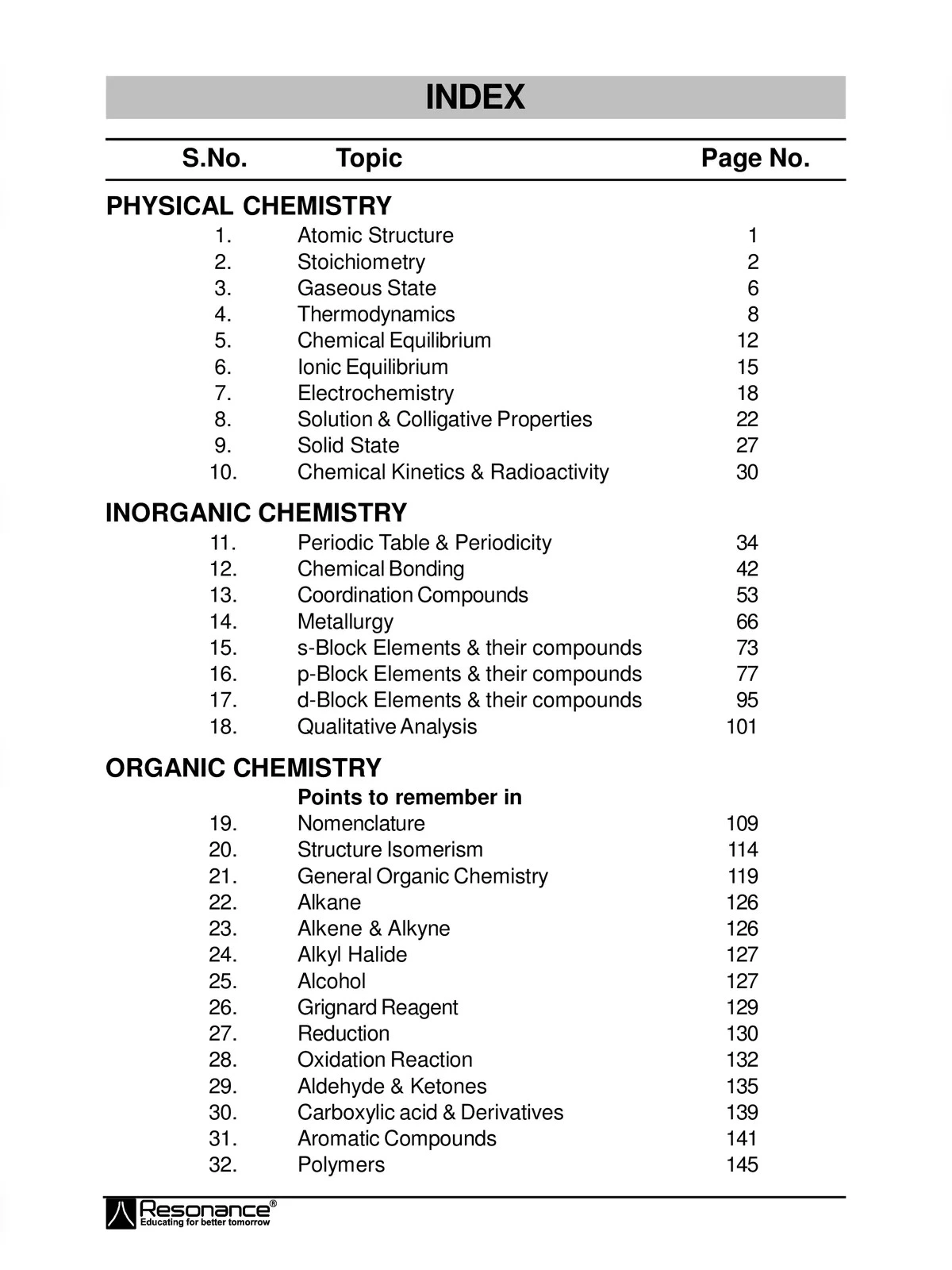
KCET Chemistry Formulas
The KCET entrance exam is organized to provide eligible candidates admission to different undergraduate courses in Karnataka. The examination is also called Karnataka CET, Kar CET, KEA UGCET or K-CET. Professional courses provided under this examination are B.Tech, B.E, B. Pharm, B.Arch and BSc.
Candidates must have completed 2nd PUC/12th Standard or an equivalent examination with a minimum of 45% Marks in Physics and Mathematics along with Chemistry/ Biology/Biotechnology/Electronics/Computer with (40% for the reserved category).
List of KCET Chemistry Formulas
- Atomic Structure:
- Atomic number (Z) = Number of protons
- Mass number (A) = Number of protons + Number of neutrons
- Number of neutrons = Mass number – Atomic number
- Number of electrons = Atomic number (for neutral atoms)
- Chemical Bonding:
- Lewis dot structure
- Ionic bond: Formed by the transfer of electrons
- Covalent bond: Formed by the sharing of electrons
- Electronegativity: Ability of an atom to attract electrons
- Chemical Equations:
- Balancing chemical equations
- Stoichiometry: Calculation of reactants and products in chemical reactions
- States of Matter:
- Ideal Gas Law: PV = nRT (Pressure * Volume = moles * gas constant * temperature)
- Boyle’s Law: PV = constant (Pressure * Volume = constant)
- Charles’ Law: V/T = constant (Volume / Temperature = constant)
- Avogadro’s Law: V/n = constant (Volume / Moles = constant)
- Thermodynamics:
- Enthalpy change (ΔH)
- Entropy (ΔS)
- Gibbs Free Energy (ΔG)
- Hess’s Law
- Chemical Kinetics:
- Rate of reaction
- Order of reaction
- Rate constant (k)
- Activation energy (Ea)
- Electrochemistry:
- Faraday’s laws of electrolysis
- Nernst equation
- Electrochemical cell notation
- Standard electrode potential (E°)
- Acids and Bases:
- pH scale
- Acid-base titrations
- Henderson-Hasselbalch equation
- Organic Chemistry:
- Functional groups
- Nomenclature of organic compounds
- Isomerism
- Reactions of organic compounds (e.g., substitution, addition, elimination)
- Periodic Table:
- Trends: Atomic radius, ionization energy, electron affinity, electronegativity