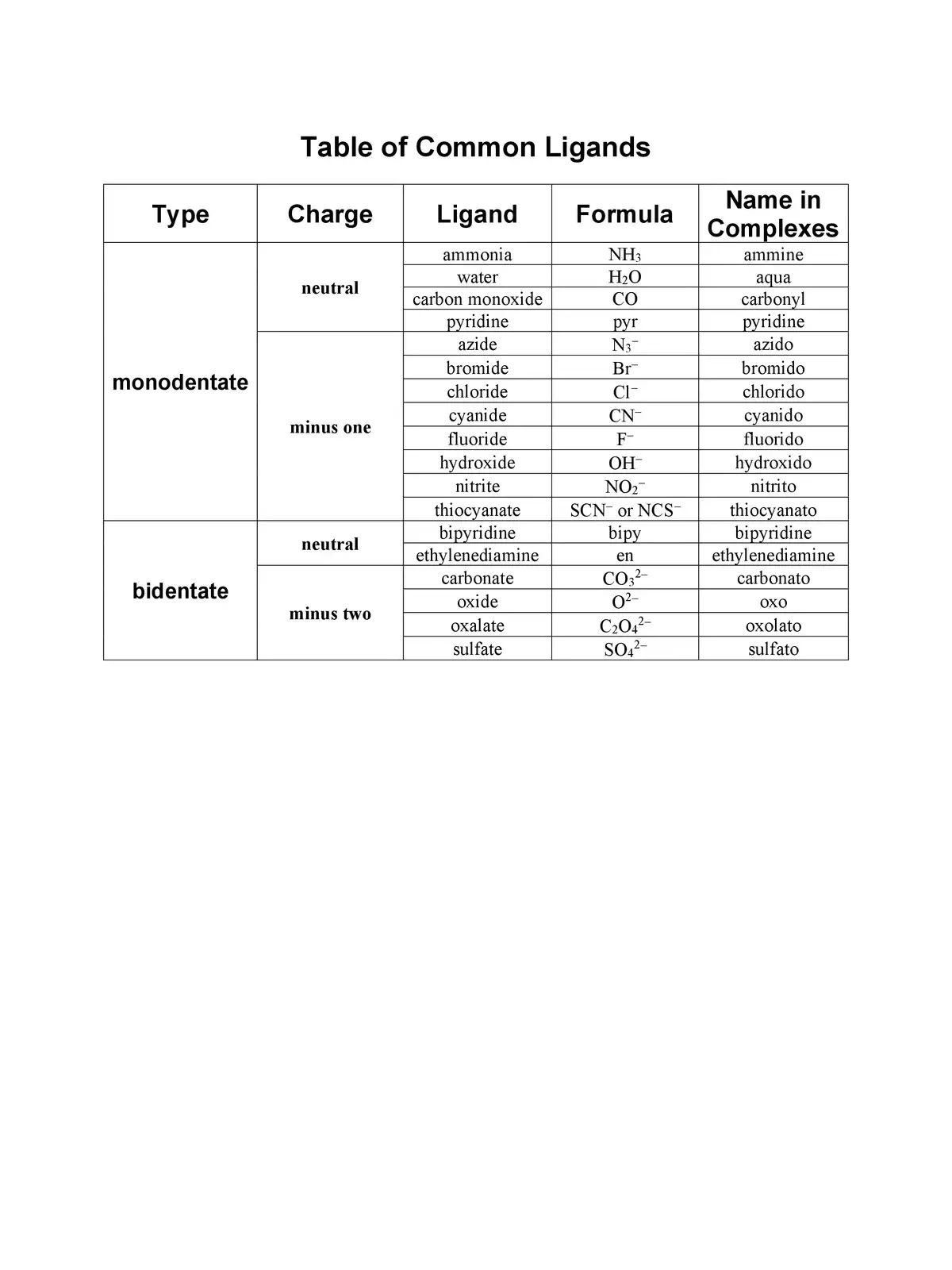
List of Ligands and Their Charges
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to create a coordination complex. This process often involves the formal donation of one or more electron pairs from the ligand. The type of bonding that occurs between the metal and the ligand can range from covalent to ionic. Additionally, the bond order between the metal and ligand can vary from one to three. Typically, ligands act as Lewis bases, although there are unusual instances where Lewis acidic “ligands” are involved.
Understanding Ligands and Their Importance
Metals and metalloids are almost always associated with ligands, except in specific conditions like high vacuum, where “naked” metal ions may occur. The ligands in a complex greatly influence the behavior of the central atom, affecting factors such as ligand substitution rates and the reactivity of the ligands, as well as important redox reactions. Choosing the right ligand is crucial in many fields, including bioinorganic chemistry, medicinal chemistry, homogeneous catalysis, and environmental science.
Download Your PDF Resource
You can download the List of Ligands and Charges in PDF format using the link provided below. This resource will help you dive deeper into the world of ligands and their various roles in coordination chemistry. Get your copy now and expand your knowledge!