53 Medicine Failed List
The pharmaceutical companies denied responsibility for these drugs. They claim they are “spurious,” and one response from a company stated, “The actual manufacturer (as per label claim) has informed that the impugned batch of the product has not been manufactured by them and that it is a spurious drug. The product is purported to be spurious; however, the same is subjected to outcome of investigation.”
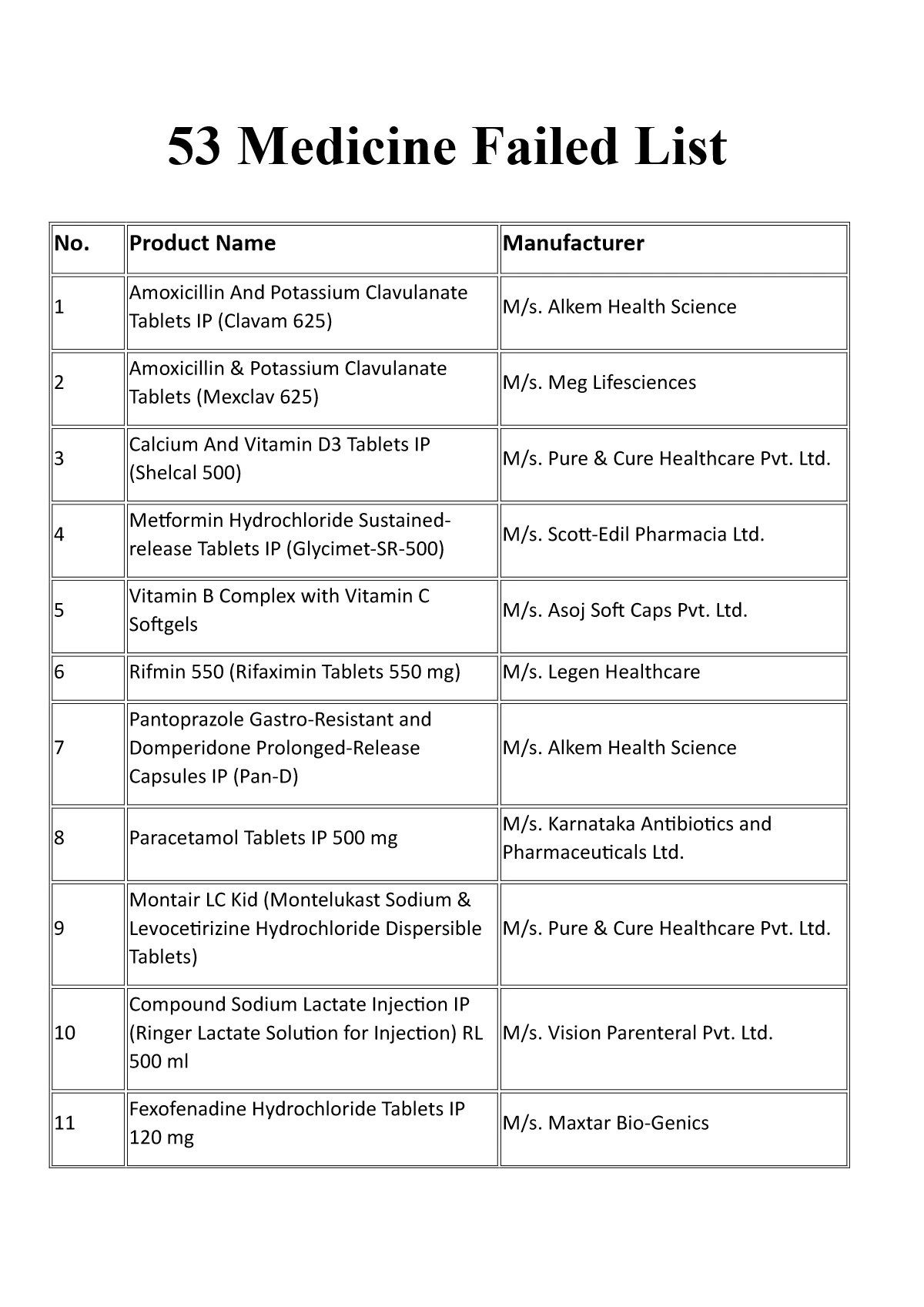
53 Medicine (Drugs) Failed List CDSCO
| S. No. | Product Name | Manufacturer |
| 1 | Amoxicillin And Potassium Clavulanate Tablets IP (Clavam 625) | M/s. Alkem Health Science |
| 2 | Amoxicillin & Potassium Clavulanate Tablets (Mexclav 625) | M/s. Meg Lifesciences |
| 3 | Calcium And Vitamin D3 Tablets IP (Shelcal 500) | M/s. Pure & Cure Healthcare Pvt. Ltd. |
| 4 | Metformin Hydrochloride Sustained-release Tablets IP (Glycimet-SR-500) | M/s. Scott-Edil Pharmacia Ltd. |
| 5 | Vitamin B Complex with Vitamin C Softgels | M/s. Asoj Soft Caps Pvt. Ltd. |
| 6 | Rifmin 550 (Rifaximin Tablets 550 mg) | M/s. Legen Healthcare |
| 7 | Pantoprazole Gastro-Resistant and Domperidone Prolonged-Release Capsules IP (Pan-D) | M/s. Alkem Health Science |
| 8 | Paracetamol Tablets IP 500 mg | M/s. Karnataka Antibiotics and Pharmaceuticals Ltd. |
| 9 | Montair LC Kid (Montelukast Sodium & Levocetirizine Hydrochloride Dispersible Tablets) | M/s. Pure & Cure Healthcare Pvt. Ltd. |
| 10 | Compound Sodium Lactate Injection IP (Ringer Lactate Solution for Injection) RL 500 ml | M/s. Vision Parenteral Pvt. Ltd. |
| 11 | Fexofenadine Hydrochloride Tablets IP 120 mg | M/s. Maxtar Bio-Genics |
| 12 | Laxnorm Solution (Lactulose Solution USP) | M/s. Athens Life Sciences |
| 13 | Heparin Sodium Injection 5000 Units (Hostranil Injection) | M/s. Health Biotech Ltd. |
| 14 | Buflam Forte Suspension (Ibuprofen & Paracetamol Oral Suspension) | M/s. Ornate Pharma Pvt. Ltd. |
| 15 | Cepodem XP 50 Dry Suspension (Cefpodoxime Proxetil and Potassium Clavulanate Oral Suspension) | M/s. Hetero Labs Limited |
| 16 | Nimesulide, Paracetamol and Chlorzoxazone Tablets (NICIP MR) | M/s. HSN International |
| 17 | Rolled Gauze (Non-Sterilized) | M/s. Blazon India |
| 18 | Ciprofloxacin Tablets IP 500 mg (Ocif-500) | M/s. Ornate Labs Pvt. Ltd. |
| 19 | Nimesulide, Phenylephrine Hydrochloride & Levocetirizine Dihydrochloride Tablets (Nunim-Cold) | M/s. Unispeed Pharmaceuticals Pvt. Ltd. |
| 20 | Adrenaline Injection IP Sterile 1 ml | M/s. Alves Healthcare Pvt. Ltd. |
| 21 | Compound Sodium Lactate Injection IP (Ringer Lactate Solution for Injection) RL 500 ml | M/s. Vision Parenteral Pvt. Ltd. |
| 22 | Vingel XL Pro Gel (Diclofenac Diethylamine, Linseed Oil, Methyl Salicylate, and Menthol Gel) | M/s. Universal Twin Labs |
| 23 | Atropine Sulphate Injection IP 2 ml | M/s. Nandani Medical Laboratories Pvt. Ltd. |
| 24 | Cefoperazone & Sulbactam For Injection (Todaycef 1.5 G) | M/s. Daxin Pharmaceuticals Pvt. Ltd. |
| 25 | Heparin Sodium Injection IP 25000 IU / 5ml | M/s. Scott-Edil Pharmacia Ltd. |
| 26 | Cefepime & Tazobactam for Injection (Crupime-TZ Kid Injection) | M/s. Cosmas Research Lab. Ltd. |
| 27 | Atropine Sulphate Injection IP (Atropine Sulphate) | M/s. Priya Pharmaceuticals |
| 28 | Salbutamol, Bromhexine HCI, Guaifenesin and Menthol Syrup (Acozil Expectorant) | M/s. Antila Lifesciences Pvt. Ltd. |
| 29 | Diclofenac Sodium IP | M/s. Sara Exports Ltd. |
| 30 | Escitalopram and Clonazepam Tablets IP (Klozaps-ES Tablets) | M/s. Digital Vision |
| 31 | Phenytoin Sodium Injection USP | M/s. Health Biotech Ltd. |
| 32 | Paracetamol, Phenylephrine Hydrochloride and Cetirizine Hydrochloride Suspension (Cethel Cold DS Suspension) | M/s. Win Cure Pharma |
| 33 | Calcium 500 mg with Vitamin D3 250 IU Tablets IP | M/s. Life Max Cancer Laboratories |
| 34 | Amoxycillin and Potassium Clavulanate Tablets IP 625 mg (Renamega-CV 625) | M/s. Malik Lifesciences Pvt. Ltd. |
| 35 | Olmesartan Medoxomil Tablets IP 40 mg | M/s. Life Max Cancer Laboratories |
| 36 | INFUSION SET-NV | M/s. Medivision Healthcare |
| 37 | Telmisartan Tablets IP 40 mg | M/s. Life Max Cancer Laboratories |
| 38 | Alprazolam Tablets IP 0.25 mg (Erazol-0.25 Tablets) | M/s. Elikem Pharmaceuticals Pvt. Ltd. |
| 39 | Glimepiride Tablets IP (2 mg) | M/s. Mascot Health Series Pvt. Ltd. |
| 40 | Calcium and Vitamin D3 Tablets IP | M/s. Unicure India Ltd. |
| 41 | Metronidazole Tablets IP 400 mg | M/s. Hindustan Antibiotics Ltd. |
| 42 | Paziva -40 | M/s. Gnosis Pharmaceuticals Pvt. Ltd. |
| 43 | Pantomed -40 | M/s. Digital Vision |
| 44 | Cefixime Oral Suspension IP (Dry Syrup) | M/s. Nestor Pharmaceuticals Ltd. |
| 45 | Moxymed CV | M/s. Alexa Pharmaceuticals |
| 46 | Frusemide Injection IP 20 mg | M/s. Nestor Pharmaceuticals Ltd. |
| 47 | Kudajarishtam | M/s. Bala Herbals |
| 48 | Tab Nodosis | M/s. Steadfast Medishield Pvt. Ltd. |
| 49 | Haridrakhanda | M/s. Bhaskara Vilasam Vaidyasala |
| 50 | Pantoprazole Inj. BP 40 mg | M/s. Kerala Medical Services Corporation Ltd. |
| 51 | Yogaraja Guggulu | M/s. Bhaskara Vilasam Vaidyasala |
| 52 | PANCEF-OF | M/s. Aglomed Ltd. |